 Drug Interaction Testing Service
Drug Interaction Testing ServiceDrug Interaction Testing Service Strategic Roadmap: Analysis and Forecasts 2025-2033
Drug Interaction Testing Service by Type (Nonclinical, Clinical), by Application (Pharmaceutical, Food), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
Key Insights
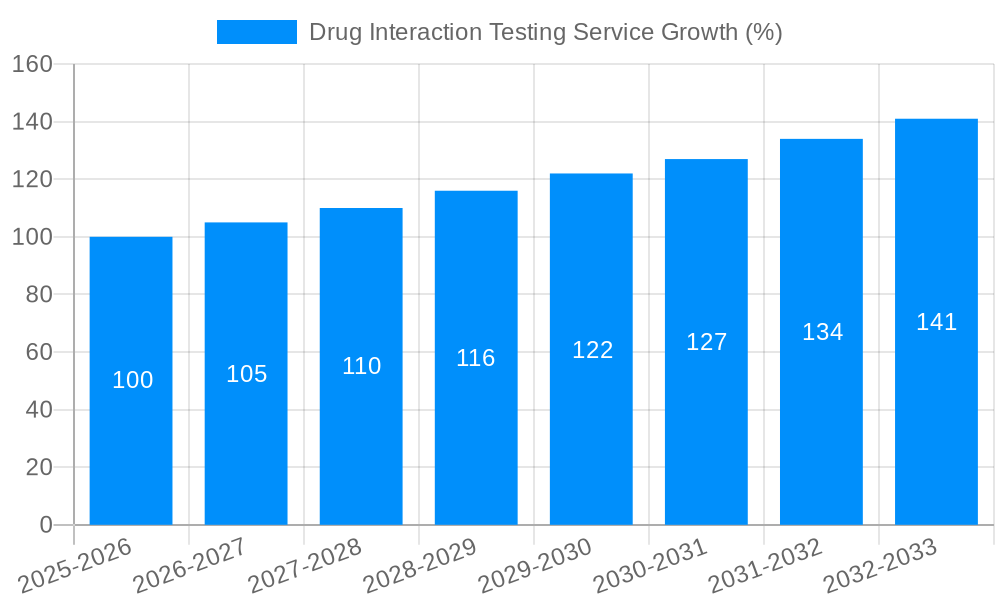
The Drug Interaction Testing Services market is experiencing steady growth, driven by the increasing number of new drug approvals and the rising need to ensure drug safety and efficacy. With a Compound Annual Growth Rate (CAGR) of 5%, the market, currently valued at approximately $2 billion in 2025, is projected to reach nearly $2.6 billion by 2033. This growth is fueled by several key factors. Firstly, stringent regulatory requirements from agencies like the FDA necessitate comprehensive drug interaction studies before market approval, creating substantial demand for these services. Secondly, the expanding pipeline of innovative therapies, including biologics and personalized medicines, is further stimulating market expansion. The pharmaceutical segment dominates the market, accounting for a significant portion of the revenue, followed by the food and non-clinical segments. North America, with its well-established pharmaceutical industry and robust regulatory framework, holds the largest market share, followed by Europe and Asia Pacific.
Despite the positive growth outlook, the market faces certain challenges. High testing costs and the complexities associated with conducting these studies can pose barriers to entry for smaller companies. Furthermore, the development and adoption of novel testing methodologies might require significant investment, presenting a short-term restraint to market expansion. However, ongoing technological advancements in in-vitro and in-vivo testing techniques are expected to alleviate this, enhancing efficiency and reducing costs in the long term. Key players in this market, including XenoTech, Nuventra Pharma Sciences, and WuXi AppTec, are actively investing in research and development to maintain their competitive edge and cater to the growing demand for sophisticated drug interaction testing services. Competition within the market is intense, driving innovation and enhancing service quality. The clinical segment is expected to witness higher growth than the non-clinical segment in the coming years, driven by increasing demand for clinical trials and phase-specific drug interaction studies.

Drug Interaction Testing Service Trends
The global drug interaction testing service market is experiencing robust growth, projected to reach multi-million dollar valuations by 2033. Driven by the increasing complexity of drug development and stringent regulatory requirements, the demand for comprehensive and reliable drug interaction studies is escalating. The market's trajectory reflects a shift towards more sophisticated in-vitro and in-vivo methodologies, a trend amplified by the rise of personalized medicine and the development of novel drug modalities, such as biologics and targeted therapies. This necessitates advanced testing services capable of accurately predicting potential drug-drug interactions (DDIs) and drug-food interactions (DFIs) to ensure patient safety and efficacy. The historical period (2019-2024) witnessed significant growth, establishing a strong foundation for the projected expansion during the forecast period (2025-2033). The estimated market value in 2025 underscores the current momentum, with considerable upward potential fueled by ongoing technological advancements and the growing pipeline of new drug candidates. This growth is further supported by the increasing outsourcing of drug interaction testing by pharmaceutical and biotechnology companies to specialized service providers, allowing them to focus on core competencies. The market is characterized by a diverse range of service providers, each offering varying levels of expertise and technological capabilities. This competitive landscape is driving innovation and improvement in the quality and efficiency of drug interaction testing services.
Driving Forces: What's Propelling the Drug Interaction Testing Service
Several key factors contribute to the expanding drug interaction testing service market. Firstly, the ever-increasing number of new drug approvals necessitates robust testing to identify and mitigate potential DDIs and DFIs. Regulatory bodies, like the FDA and EMA, enforce stringent guidelines on preclinical and clinical drug interaction studies, making these services indispensable. Secondly, the growing complexity of drug formulations and combination therapies significantly elevates the risk of unforeseen interactions, necessitating more sophisticated testing methodologies. Thirdly, the burgeoning personalized medicine field demands tailored drug interaction profiles for specific patient populations, further fueling the demand for customized testing solutions. The rising prevalence of chronic diseases necessitates the development of polypharmacy treatments, which increases the risk of DDIs and the need for comprehensive testing. Finally, the cost-effectiveness of outsourcing drug interaction testing to specialized CROs (Contract Research Organizations) allows pharmaceutical companies to optimize their resources and accelerate drug development timelines. This trend significantly contributes to the overall growth of the market.
Challenges and Restraints in Drug Interaction Testing Service
Despite the substantial growth potential, the drug interaction testing service market faces certain challenges. High costs associated with advanced testing technologies and specialized expertise can limit accessibility for smaller pharmaceutical companies. The complexity of designing and executing comprehensive interaction studies, particularly those involving complex drug combinations or patient populations with comorbidities, presents significant logistical and analytical hurdles. Furthermore, the interpretation of drug interaction study results requires a deep understanding of pharmacokinetics and pharmacodynamics, which necessitates highly skilled personnel. Harmonizing global regulatory requirements for drug interaction studies can also prove challenging for service providers operating across different jurisdictions. Ensuring data integrity and reproducibility across different testing labs is another critical issue that needs meticulous attention to maintain regulatory compliance and scientific rigor. The evolving regulatory landscape and continuous updates to testing guidelines also require ongoing investment in training and technology upgrades for service providers to remain competitive.
Key Region or Country & Segment to Dominate the Market
The North American region, particularly the United States, is expected to maintain its dominance in the drug interaction testing service market due to the high concentration of pharmaceutical companies, robust regulatory frameworks, and substantial investment in research and development. Within the segments, the pharmaceutical application segment is projected to hold the largest market share. This dominance stems from the large number of pharmaceutical companies actively involved in drug discovery and development.
- North America: High concentration of pharmaceutical and biotechnology companies, robust regulatory framework, significant R&D investment.
- Europe: Stringent regulatory standards, growing focus on personalized medicine, increasing number of clinical trials.
- Asia-Pacific: Rapid growth in the pharmaceutical industry, rising healthcare expenditure, and increasing awareness of drug safety.
- Pharmaceutical Application: This segment leads due to the high volume of new drug development and the stringent regulatory requirements for drug interaction studies within the pharmaceutical industry. This segment is the primary driver of demand for comprehensive drug interaction testing services. The increasing complexity of drug formulations, combined with the rising incidence of polypharmacy, only serves to increase the requirement for detailed drug interaction testing within this segment.
The non-clinical segment also holds substantial potential, as preclinical drug interaction studies are a crucial step in the drug development process, providing valuable information for designing safe and effective clinical trials. The demand for these services continues to grow with the influx of new drugs into development pipelines.
Growth Catalysts in Drug Interaction Testing Service Industry
The market is propelled by several catalysts including the increasing demand for personalized medicine which necessitates tailored drug interaction assessments, stringent regulatory requirements for DDI and DFI studies, rising prevalence of chronic diseases leading to polypharmacy, and the cost-effectiveness of outsourcing drug interaction testing to specialized CROs. These factors collectively create a significant market opportunity.
Leading Players in the Drug Interaction Testing Service
- XenoTech
- Nuventra Pharma Sciences
- TIM
- Creative Biolabs
- Aegis Sciences Corporation
- Admescope
- Corning
- Creative Bioarray
- Cyprotex
- Worldwide Clinical Trials
- Alcami
- IQVIA
- WuXi AppTec
Significant Developments in Drug Interaction Testing Service Sector
- 2020: XenoTech launched a new high-throughput screening platform for drug interaction studies.
- 2021: Aegis Sciences Corporation expanded its clinical testing capabilities to include advanced DDI analysis.
- 2022: Nuventra Pharma Sciences secured a significant contract for a large-scale drug interaction study.
- 2023: Several companies invested in advanced in-silico modeling techniques to enhance prediction accuracy and reduce testing costs. (Specific company names withheld for brevity).
Comprehensive Coverage Drug Interaction Testing Service Report
This report provides a detailed overview of the drug interaction testing service market, encompassing market size estimations, growth forecasts, segment analysis, key market trends, competitive landscape, and significant industry developments. The study covers the historical period (2019-2024), base year (2025), estimated year (2025), and forecast period (2025-2033) providing a comprehensive perspective on the market's trajectory. The analysis encompasses both qualitative and quantitative insights to offer a holistic understanding of the market dynamics.
Drug Interaction Testing Service Segmentation
-
1. Type
- 1.1. Nonclinical
- 1.2. Clinical
-
2. Application
- 2.1. Pharmaceutical
- 2.2. Food
Drug Interaction Testing Service Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific

Drug Interaction Testing Service REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 5% from 2019-2033 |
| Segmentation |
|
Frequently Asked Questions
What are the notable trends driving market growth?
.
What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00 , USD 5220.00, and USD 6960.00 respectively.
Can you provide details about the market size?
The market size is estimated to be USD XXX million as of 2022.
Are there any additional resources or data provided in the report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
What are the main segments of the Drug Interaction Testing Service?
The market segments include
What are some drivers contributing to market growth?
.
Are there any restraints impacting market growth?
.
Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million .
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Drug Interaction Testing Service Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Type
- 5.1.1. Nonclinical
- 5.1.2. Clinical
- 5.2. Market Analysis, Insights and Forecast - by Application
- 5.2.1. Pharmaceutical
- 5.2.2. Food
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Type
- 6. North America Drug Interaction Testing Service Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Type
- 6.1.1. Nonclinical
- 6.1.2. Clinical
- 6.2. Market Analysis, Insights and Forecast - by Application
- 6.2.1. Pharmaceutical
- 6.2.2. Food
- 6.1. Market Analysis, Insights and Forecast - by Type
- 7. South America Drug Interaction Testing Service Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Type
- 7.1.1. Nonclinical
- 7.1.2. Clinical
- 7.2. Market Analysis, Insights and Forecast - by Application
- 7.2.1. Pharmaceutical
- 7.2.2. Food
- 7.1. Market Analysis, Insights and Forecast - by Type
- 8. Europe Drug Interaction Testing Service Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Type
- 8.1.1. Nonclinical
- 8.1.2. Clinical
- 8.2. Market Analysis, Insights and Forecast - by Application
- 8.2.1. Pharmaceutical
- 8.2.2. Food
- 8.1. Market Analysis, Insights and Forecast - by Type
- 9. Middle East & Africa Drug Interaction Testing Service Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Type
- 9.1.1. Nonclinical
- 9.1.2. Clinical
- 9.2. Market Analysis, Insights and Forecast - by Application
- 9.2.1. Pharmaceutical
- 9.2.2. Food
- 9.1. Market Analysis, Insights and Forecast - by Type
- 10. Asia Pacific Drug Interaction Testing Service Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Type
- 10.1.1. Nonclinical
- 10.1.2. Clinical
- 10.2. Market Analysis, Insights and Forecast - by Application
- 10.2.1. Pharmaceutical
- 10.2.2. Food
- 10.1. Market Analysis, Insights and Forecast - by Type
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 XenoTech
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Nuventra Pharma Sciences
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 TIM
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Creative Biolabs
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Aegis Sciences Corporation
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Admescope
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Corning
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Creative Bioarray
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Cyprotex
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Worldwide Clinical Trials
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Alcami
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 IQVIA
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.13 WuXi AppTec
- 11.2.13.1. Overview
- 11.2.13.2. Products
- 11.2.13.3. SWOT Analysis
- 11.2.13.4. Recent Developments
- 11.2.13.5. Financials (Based on Availability)
- 11.2.14
- 11.2.14.1. Overview
- 11.2.14.2. Products
- 11.2.14.3. SWOT Analysis
- 11.2.14.4. Recent Developments
- 11.2.14.5. Financials (Based on Availability)
- 11.2.1 XenoTech
- Figure 1: Global Drug Interaction Testing Service Revenue Breakdown (million, %) by Region 2024 & 2032
- Figure 2: North America Drug Interaction Testing Service Revenue (million), by Type 2024 & 2032
- Figure 3: North America Drug Interaction Testing Service Revenue Share (%), by Type 2024 & 2032
- Figure 4: North America Drug Interaction Testing Service Revenue (million), by Application 2024 & 2032
- Figure 5: North America Drug Interaction Testing Service Revenue Share (%), by Application 2024 & 2032
- Figure 6: North America Drug Interaction Testing Service Revenue (million), by Country 2024 & 2032
- Figure 7: North America Drug Interaction Testing Service Revenue Share (%), by Country 2024 & 2032
- Figure 8: South America Drug Interaction Testing Service Revenue (million), by Type 2024 & 2032
- Figure 9: South America Drug Interaction Testing Service Revenue Share (%), by Type 2024 & 2032
- Figure 10: South America Drug Interaction Testing Service Revenue (million), by Application 2024 & 2032
- Figure 11: South America Drug Interaction Testing Service Revenue Share (%), by Application 2024 & 2032
- Figure 12: South America Drug Interaction Testing Service Revenue (million), by Country 2024 & 2032
- Figure 13: South America Drug Interaction Testing Service Revenue Share (%), by Country 2024 & 2032
- Figure 14: Europe Drug Interaction Testing Service Revenue (million), by Type 2024 & 2032
- Figure 15: Europe Drug Interaction Testing Service Revenue Share (%), by Type 2024 & 2032
- Figure 16: Europe Drug Interaction Testing Service Revenue (million), by Application 2024 & 2032
- Figure 17: Europe Drug Interaction Testing Service Revenue Share (%), by Application 2024 & 2032
- Figure 18: Europe Drug Interaction Testing Service Revenue (million), by Country 2024 & 2032
- Figure 19: Europe Drug Interaction Testing Service Revenue Share (%), by Country 2024 & 2032
- Figure 20: Middle East & Africa Drug Interaction Testing Service Revenue (million), by Type 2024 & 2032
- Figure 21: Middle East & Africa Drug Interaction Testing Service Revenue Share (%), by Type 2024 & 2032
- Figure 22: Middle East & Africa Drug Interaction Testing Service Revenue (million), by Application 2024 & 2032
- Figure 23: Middle East & Africa Drug Interaction Testing Service Revenue Share (%), by Application 2024 & 2032
- Figure 24: Middle East & Africa Drug Interaction Testing Service Revenue (million), by Country 2024 & 2032
- Figure 25: Middle East & Africa Drug Interaction Testing Service Revenue Share (%), by Country 2024 & 2032
- Figure 26: Asia Pacific Drug Interaction Testing Service Revenue (million), by Type 2024 & 2032
- Figure 27: Asia Pacific Drug Interaction Testing Service Revenue Share (%), by Type 2024 & 2032
- Figure 28: Asia Pacific Drug Interaction Testing Service Revenue (million), by Application 2024 & 2032
- Figure 29: Asia Pacific Drug Interaction Testing Service Revenue Share (%), by Application 2024 & 2032
- Figure 30: Asia Pacific Drug Interaction Testing Service Revenue (million), by Country 2024 & 2032
- Figure 31: Asia Pacific Drug Interaction Testing Service Revenue Share (%), by Country 2024 & 2032
- Table 1: Global Drug Interaction Testing Service Revenue million Forecast, by Region 2019 & 2032
- Table 2: Global Drug Interaction Testing Service Revenue million Forecast, by Type 2019 & 2032
- Table 3: Global Drug Interaction Testing Service Revenue million Forecast, by Application 2019 & 2032
- Table 4: Global Drug Interaction Testing Service Revenue million Forecast, by Region 2019 & 2032
- Table 5: Global Drug Interaction Testing Service Revenue million Forecast, by Type 2019 & 2032
- Table 6: Global Drug Interaction Testing Service Revenue million Forecast, by Application 2019 & 2032
- Table 7: Global Drug Interaction Testing Service Revenue million Forecast, by Country 2019 & 2032
- Table 8: United States Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 9: Canada Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 10: Mexico Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 11: Global Drug Interaction Testing Service Revenue million Forecast, by Type 2019 & 2032
- Table 12: Global Drug Interaction Testing Service Revenue million Forecast, by Application 2019 & 2032
- Table 13: Global Drug Interaction Testing Service Revenue million Forecast, by Country 2019 & 2032
- Table 14: Brazil Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 15: Argentina Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 16: Rest of South America Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 17: Global Drug Interaction Testing Service Revenue million Forecast, by Type 2019 & 2032
- Table 18: Global Drug Interaction Testing Service Revenue million Forecast, by Application 2019 & 2032
- Table 19: Global Drug Interaction Testing Service Revenue million Forecast, by Country 2019 & 2032
- Table 20: United Kingdom Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 21: Germany Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 22: France Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 23: Italy Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 24: Spain Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 25: Russia Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 26: Benelux Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 27: Nordics Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 28: Rest of Europe Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 29: Global Drug Interaction Testing Service Revenue million Forecast, by Type 2019 & 2032
- Table 30: Global Drug Interaction Testing Service Revenue million Forecast, by Application 2019 & 2032
- Table 31: Global Drug Interaction Testing Service Revenue million Forecast, by Country 2019 & 2032
- Table 32: Turkey Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 33: Israel Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 34: GCC Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 35: North Africa Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 36: South Africa Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 37: Rest of Middle East & Africa Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 38: Global Drug Interaction Testing Service Revenue million Forecast, by Type 2019 & 2032
- Table 39: Global Drug Interaction Testing Service Revenue million Forecast, by Application 2019 & 2032
- Table 40: Global Drug Interaction Testing Service Revenue million Forecast, by Country 2019 & 2032
- Table 41: China Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 42: India Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 43: Japan Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 44: South Korea Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 45: ASEAN Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 46: Oceania Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
- Table 47: Rest of Asia Pacific Drug Interaction Testing Service Revenue (million) Forecast, by Application 2019 & 2032
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 5% from 2019-2033 |
| Segmentation |
|
STEP 1 - Identification of Relevant Samples Size from Population Database



STEP 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note* : In applicable scenarios
STEP 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

STEP 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
Related Reports
About Market Research Forecast
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.

