 Inhalation CDMO
Inhalation CDMOInhalation CDMO Report Probes the 6049 million Size, Share, Growth Report and Future Analysis by 2033
Inhalation CDMO by Type (Metered Dose Inhalers (MDIs), Dry Powder Inhalers (DPIs), Soft Mist Inhalers (SMIs), Others), by Application (Commercial, Academic Research, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
Key Insights
The Inhalation CDMO market, valued at $6,049 million in 2025, is projected to experience robust growth, driven by a compound annual growth rate (CAGR) of 10.9% from 2025 to 2033. This expansion is fueled by several key factors. Firstly, the increasing prevalence of respiratory diseases globally, including asthma, COPD, and cystic fibrosis, necessitates a higher demand for inhaled drug products. Secondly, the rising adoption of advanced inhalation technologies, such as dry powder inhalers (DPIs) and soft mist inhalers (SMIs), offering improved drug delivery and patient compliance, further boosts market growth. Technological advancements in formulation development and manufacturing processes, enabling the creation of more efficient and patient-friendly inhalers, also contribute significantly. Finally, a growing number of pharmaceutical and biotechnology companies are outsourcing their inhalation drug development and manufacturing to CDMOs, leading to increased market demand. This trend is particularly noticeable in North America and Europe, which currently hold the largest market shares due to established research infrastructure, regulatory frameworks, and strong pharmaceutical industries.
The Inhalation CDMO market's segmentation reveals Metered Dose Inhalers (MDIs) currently dominate, but the Dry Powder Inhalers (DPIs) and Soft Mist Inhalers (SMIs) segments are expected to exhibit faster growth rates over the forecast period. The commercial application segment holds a significant share, with academic research also contributing substantially. Competition among established players such as Lonza, Vectura, and Catalent, as well as emerging players like Iconovo and Kindeva, is intense, driving innovation and fostering advancements in technology and service offerings. Geographic expansion, particularly in rapidly developing economies of Asia-Pacific and emerging markets in the Middle East & Africa, will likely represent a significant growth opportunity. However, regulatory hurdles, stringent quality control requirements, and the high capital investment needed for specialized equipment pose potential challenges to market growth.
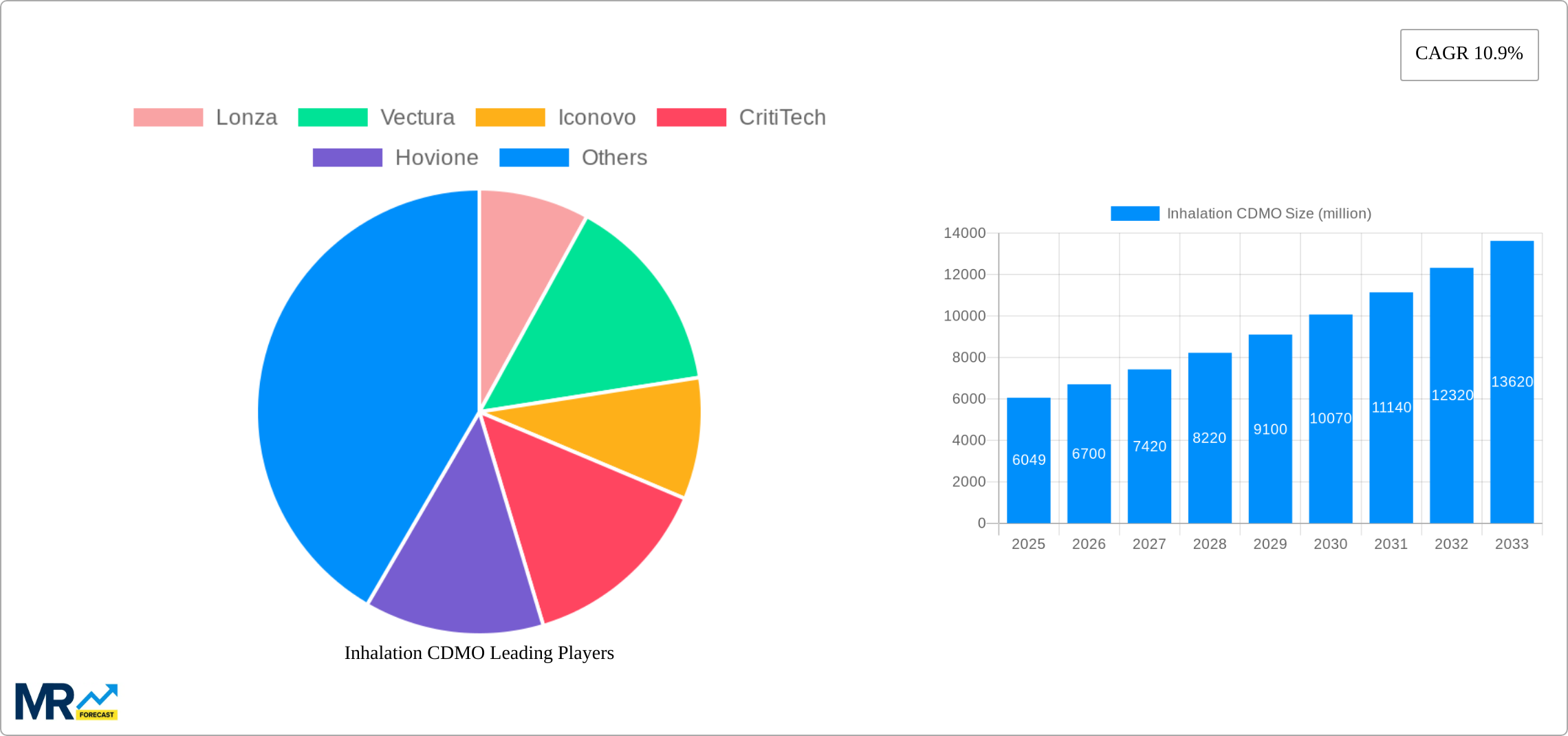
Inhalation CDMO Trends
The inhalation CDMO (Contract Development and Manufacturing Organization) market is experiencing robust growth, projected to reach USD XXX million by 2033, expanding at a CAGR of XX% during the forecast period (2025-2033). The historical period (2019-2024) saw significant expansion driven by the increasing prevalence of respiratory diseases globally, a rising demand for innovative inhalation drug delivery systems, and a growing outsourcing trend within the pharmaceutical industry. The base year for this analysis is 2025, with estimations for that year already indicating a market value of USD XXX million. This growth is fueled by the complexity and specialized expertise required for inhalation product development and manufacturing, pushing pharmaceutical companies to partner with CDMOs possessing the necessary infrastructure and technological capabilities. Key market insights reveal a strong preference for DPIs (Dry Powder Inhalers) due to their ease of use and patient compliance, while MDIs (Metered Dose Inhalers) maintain a substantial market share due to their established presence and technological maturity. The increasing adoption of personalized medicine and the development of advanced formulations further contribute to the market’s dynamic growth trajectory. The market is witnessing a surge in demand for services across the entire drug development lifecycle, from formulation development and analytical testing to clinical trial supplies and commercial manufacturing. This comprehensive approach by CDMOs is a key factor in their success and the continued expansion of this critical sector within the pharmaceutical industry. The competitive landscape is marked by both large established players and smaller specialized firms, all vying for market share through innovative solutions and strategic partnerships.
Driving Forces: What's Propelling the Inhalation CDMO Market?
Several factors are driving the expansion of the inhalation CDMO market. The surging prevalence of respiratory diseases like asthma, COPD, and cystic fibrosis necessitates the development of increasingly sophisticated and effective inhalation therapies. This demand fuels the need for specialized CDMOs capable of handling the intricate processes involved in manufacturing these products. Furthermore, pharmaceutical companies are increasingly outsourcing their development and manufacturing activities to CDMOs, leveraging their expertise and infrastructure to streamline their operations and reduce costs. This outsourcing trend is particularly pronounced in the inhalation space due to the high regulatory hurdles and complex manufacturing processes associated with inhalation drug products. The rise of novel drug delivery systems, such as SMIs (Soft Mist Inhalers) and next-generation DPI technologies, also presents significant growth opportunities for CDMOs. These advancements offer improved patient compliance and efficacy, driving further market expansion. Moreover, the growing emphasis on personalized medicine, requiring tailored drug formulations, is leading pharmaceutical companies to seek partnerships with CDMOs that can meet their specific needs. The increasing investment in research and development within the pharmaceutical sector, aimed at discovering and developing new inhalation therapies, further reinforces the growth prospects for inhalation CDMOs.

Challenges and Restraints in Inhalation CDMO
Despite the significant growth potential, the inhalation CDMO market faces several challenges. Stringent regulatory requirements and compliance standards for inhalation drug products necessitate substantial investment in quality control and assurance measures, adding to operational costs. The complexity of manufacturing processes for inhalation products, particularly the need for precise particle size control and efficient drug delivery, can lead to higher manufacturing costs and longer lead times. Competition among CDMOs is intense, with both large multinational companies and smaller specialized firms vying for market share. This competitive landscape necessitates continuous innovation and investment in advanced technologies to maintain a competitive edge. Furthermore, securing skilled personnel with expertise in inhalation drug development and manufacturing poses a challenge for many CDMOs. The fluctuating price of raw materials and the dependence on specialized equipment and technologies can impact profitability and create operational uncertainties. Maintaining a high level of quality control and compliance while scaling up production to meet increasing demand is also a critical hurdle for CDMOs in this sector.
Key Region or Country & Segment to Dominate the Market
The Dry Powder Inhaler (DPI) segment is poised to dominate the market over the forecast period.
Reasons for DPI Dominance: DPIs offer several advantages compared to MDIs, including ease of use, reduced propellant dependence, and better patient compliance. Technological advancements in DPI design and manufacturing further enhance their appeal. The growing adoption of DPI devices in the treatment of various respiratory diseases significantly contributes to this segment's dominance.
Geographic Dominance: North America and Europe are expected to hold significant market share during the forecast period. These regions boast a robust pharmaceutical industry, a high prevalence of respiratory diseases, and a strong regulatory framework supportive of innovation in drug delivery systems. The presence of several established CDMOs in these regions also contributes to their dominance.
Further Breakdown:
- North America: Strong regulatory support, established pharmaceutical infrastructure, and high healthcare expenditure drive growth.
- Europe: Significant investments in pharmaceutical R&D, presence of major CDMO players, and high prevalence of respiratory diseases contribute to market dominance.
- Asia Pacific: This region is witnessing rapid growth in the prevalence of respiratory diseases and increasing healthcare expenditure. However, regulatory hurdles and infrastructural limitations might slow growth compared to North America and Europe.
The Commercial application segment is also expected to represent a substantial market share due to the high volume of commercial drug manufacturing for respiratory treatments.
Growth Catalysts in the Inhalation CDMO Industry
The inhalation CDMO industry is experiencing robust growth due to a confluence of factors. Increasing prevalence of respiratory diseases worldwide creates substantial demand for effective treatments. Simultaneously, the pharmaceutical industry's ongoing trend towards outsourcing drug development and manufacturing accelerates CDMO growth. The development of novel drug delivery systems such as SMIs and next-generation DPIs, alongside ongoing advancements in formulation technologies, further fuels market expansion.
Leading Players in the Inhalation CDMO Market
- Lonza
- Vectura
- Iconovo
- CritiTech
- Hovione
- Recipharm
- Aptar Pharma
- Kindeva
- Sanner
- Particle Sciences
- Experic
- Enteris Biopharma
- Catalent
- HCmed
- Ritedose
- Bespak
- Proveris
- Bend Bioscience
- Renejix
Significant Developments in the Inhalation CDMO Sector
- 2021: Lonza invests in expanding its inhalation capabilities.
- 2022: Vectura acquires a new facility specializing in DPI manufacturing.
- 2023: Catalent announces a strategic partnership with a leading pharmaceutical company for inhalation product development.
- 2024: Hovione expands its capacity to produce advanced inhalation formulations. (Note: These are examples and would need to be replaced with actual significant developments from the period.)
Comprehensive Coverage Inhalation CDMO Report
This report provides a detailed analysis of the inhalation CDMO market, covering key trends, driving forces, challenges, and growth catalysts. It offers in-depth segment analysis and regional market insights, along with profiles of leading industry players and their significant developments. The report's comprehensive coverage offers valuable information for stakeholders seeking to understand and navigate this dynamic market. The extensive data analysis coupled with expert market insights facilitates strategic decision-making and informed investment strategies in the inhalation CDMO sector.
Inhalation CDMO Segmentation
-
1. Type
- 1.1. Metered Dose Inhalers (MDIs)
- 1.2. Dry Powder Inhalers (DPIs)
- 1.3. Soft Mist Inhalers (SMIs)
- 1.4. Others
-
2. Application
- 2.1. Commercial
- 2.2. Academic Research
- 2.3. Others
Inhalation CDMO Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific

Inhalation CDMO REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 10.9% from 2019-2033 |
| Segmentation |
|
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Inhalation CDMO Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Type
- 5.1.1. Metered Dose Inhalers (MDIs)
- 5.1.2. Dry Powder Inhalers (DPIs)
- 5.1.3. Soft Mist Inhalers (SMIs)
- 5.1.4. Others
- 5.2. Market Analysis, Insights and Forecast - by Application
- 5.2.1. Commercial
- 5.2.2. Academic Research
- 5.2.3. Others
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Type
- 6. North America Inhalation CDMO Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Type
- 6.1.1. Metered Dose Inhalers (MDIs)
- 6.1.2. Dry Powder Inhalers (DPIs)
- 6.1.3. Soft Mist Inhalers (SMIs)
- 6.1.4. Others
- 6.2. Market Analysis, Insights and Forecast - by Application
- 6.2.1. Commercial
- 6.2.2. Academic Research
- 6.2.3. Others
- 6.1. Market Analysis, Insights and Forecast - by Type
- 7. South America Inhalation CDMO Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Type
- 7.1.1. Metered Dose Inhalers (MDIs)
- 7.1.2. Dry Powder Inhalers (DPIs)
- 7.1.3. Soft Mist Inhalers (SMIs)
- 7.1.4. Others
- 7.2. Market Analysis, Insights and Forecast - by Application
- 7.2.1. Commercial
- 7.2.2. Academic Research
- 7.2.3. Others
- 7.1. Market Analysis, Insights and Forecast - by Type
- 8. Europe Inhalation CDMO Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Type
- 8.1.1. Metered Dose Inhalers (MDIs)
- 8.1.2. Dry Powder Inhalers (DPIs)
- 8.1.3. Soft Mist Inhalers (SMIs)
- 8.1.4. Others
- 8.2. Market Analysis, Insights and Forecast - by Application
- 8.2.1. Commercial
- 8.2.2. Academic Research
- 8.2.3. Others
- 8.1. Market Analysis, Insights and Forecast - by Type
- 9. Middle East & Africa Inhalation CDMO Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Type
- 9.1.1. Metered Dose Inhalers (MDIs)
- 9.1.2. Dry Powder Inhalers (DPIs)
- 9.1.3. Soft Mist Inhalers (SMIs)
- 9.1.4. Others
- 9.2. Market Analysis, Insights and Forecast - by Application
- 9.2.1. Commercial
- 9.2.2. Academic Research
- 9.2.3. Others
- 9.1. Market Analysis, Insights and Forecast - by Type
- 10. Asia Pacific Inhalation CDMO Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Type
- 10.1.1. Metered Dose Inhalers (MDIs)
- 10.1.2. Dry Powder Inhalers (DPIs)
- 10.1.3. Soft Mist Inhalers (SMIs)
- 10.1.4. Others
- 10.2. Market Analysis, Insights and Forecast - by Application
- 10.2.1. Commercial
- 10.2.2. Academic Research
- 10.2.3. Others
- 10.1. Market Analysis, Insights and Forecast - by Type
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 Lonza
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Vectura
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Iconovo
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 CritiTech
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Hovione
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Recipharm
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Aptar Pharma
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Kindeva
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Sanner
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Particle Sciences
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Experic
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Enteris Biopharma
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.13 Catalent
- 11.2.13.1. Overview
- 11.2.13.2. Products
- 11.2.13.3. SWOT Analysis
- 11.2.13.4. Recent Developments
- 11.2.13.5. Financials (Based on Availability)
- 11.2.14 HCmed
- 11.2.14.1. Overview
- 11.2.14.2. Products
- 11.2.14.3. SWOT Analysis
- 11.2.14.4. Recent Developments
- 11.2.14.5. Financials (Based on Availability)
- 11.2.15 Ritedose
- 11.2.15.1. Overview
- 11.2.15.2. Products
- 11.2.15.3. SWOT Analysis
- 11.2.15.4. Recent Developments
- 11.2.15.5. Financials (Based on Availability)
- 11.2.16 Bespak
- 11.2.16.1. Overview
- 11.2.16.2. Products
- 11.2.16.3. SWOT Analysis
- 11.2.16.4. Recent Developments
- 11.2.16.5. Financials (Based on Availability)
- 11.2.17 Proveris
- 11.2.17.1. Overview
- 11.2.17.2. Products
- 11.2.17.3. SWOT Analysis
- 11.2.17.4. Recent Developments
- 11.2.17.5. Financials (Based on Availability)
- 11.2.18 Bend Bioscience
- 11.2.18.1. Overview
- 11.2.18.2. Products
- 11.2.18.3. SWOT Analysis
- 11.2.18.4. Recent Developments
- 11.2.18.5. Financials (Based on Availability)
- 11.2.19 Renejix
- 11.2.19.1. Overview
- 11.2.19.2. Products
- 11.2.19.3. SWOT Analysis
- 11.2.19.4. Recent Developments
- 11.2.19.5. Financials (Based on Availability)
- 11.2.1 Lonza
- Figure 1: Global Inhalation CDMO Revenue Breakdown (million, %) by Region 2024 & 2032
- Figure 2: North America Inhalation CDMO Revenue (million), by Type 2024 & 2032
- Figure 3: North America Inhalation CDMO Revenue Share (%), by Type 2024 & 2032
- Figure 4: North America Inhalation CDMO Revenue (million), by Application 2024 & 2032
- Figure 5: North America Inhalation CDMO Revenue Share (%), by Application 2024 & 2032
- Figure 6: North America Inhalation CDMO Revenue (million), by Country 2024 & 2032
- Figure 7: North America Inhalation CDMO Revenue Share (%), by Country 2024 & 2032
- Figure 8: South America Inhalation CDMO Revenue (million), by Type 2024 & 2032
- Figure 9: South America Inhalation CDMO Revenue Share (%), by Type 2024 & 2032
- Figure 10: South America Inhalation CDMO Revenue (million), by Application 2024 & 2032
- Figure 11: South America Inhalation CDMO Revenue Share (%), by Application 2024 & 2032
- Figure 12: South America Inhalation CDMO Revenue (million), by Country 2024 & 2032
- Figure 13: South America Inhalation CDMO Revenue Share (%), by Country 2024 & 2032
- Figure 14: Europe Inhalation CDMO Revenue (million), by Type 2024 & 2032
- Figure 15: Europe Inhalation CDMO Revenue Share (%), by Type 2024 & 2032
- Figure 16: Europe Inhalation CDMO Revenue (million), by Application 2024 & 2032
- Figure 17: Europe Inhalation CDMO Revenue Share (%), by Application 2024 & 2032
- Figure 18: Europe Inhalation CDMO Revenue (million), by Country 2024 & 2032
- Figure 19: Europe Inhalation CDMO Revenue Share (%), by Country 2024 & 2032
- Figure 20: Middle East & Africa Inhalation CDMO Revenue (million), by Type 2024 & 2032
- Figure 21: Middle East & Africa Inhalation CDMO Revenue Share (%), by Type 2024 & 2032
- Figure 22: Middle East & Africa Inhalation CDMO Revenue (million), by Application 2024 & 2032
- Figure 23: Middle East & Africa Inhalation CDMO Revenue Share (%), by Application 2024 & 2032
- Figure 24: Middle East & Africa Inhalation CDMO Revenue (million), by Country 2024 & 2032
- Figure 25: Middle East & Africa Inhalation CDMO Revenue Share (%), by Country 2024 & 2032
- Figure 26: Asia Pacific Inhalation CDMO Revenue (million), by Type 2024 & 2032
- Figure 27: Asia Pacific Inhalation CDMO Revenue Share (%), by Type 2024 & 2032
- Figure 28: Asia Pacific Inhalation CDMO Revenue (million), by Application 2024 & 2032
- Figure 29: Asia Pacific Inhalation CDMO Revenue Share (%), by Application 2024 & 2032
- Figure 30: Asia Pacific Inhalation CDMO Revenue (million), by Country 2024 & 2032
- Figure 31: Asia Pacific Inhalation CDMO Revenue Share (%), by Country 2024 & 2032
- Table 1: Global Inhalation CDMO Revenue million Forecast, by Region 2019 & 2032
- Table 2: Global Inhalation CDMO Revenue million Forecast, by Type 2019 & 2032
- Table 3: Global Inhalation CDMO Revenue million Forecast, by Application 2019 & 2032
- Table 4: Global Inhalation CDMO Revenue million Forecast, by Region 2019 & 2032
- Table 5: Global Inhalation CDMO Revenue million Forecast, by Type 2019 & 2032
- Table 6: Global Inhalation CDMO Revenue million Forecast, by Application 2019 & 2032
- Table 7: Global Inhalation CDMO Revenue million Forecast, by Country 2019 & 2032
- Table 8: United States Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 9: Canada Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 10: Mexico Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 11: Global Inhalation CDMO Revenue million Forecast, by Type 2019 & 2032
- Table 12: Global Inhalation CDMO Revenue million Forecast, by Application 2019 & 2032
- Table 13: Global Inhalation CDMO Revenue million Forecast, by Country 2019 & 2032
- Table 14: Brazil Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 15: Argentina Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 16: Rest of South America Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 17: Global Inhalation CDMO Revenue million Forecast, by Type 2019 & 2032
- Table 18: Global Inhalation CDMO Revenue million Forecast, by Application 2019 & 2032
- Table 19: Global Inhalation CDMO Revenue million Forecast, by Country 2019 & 2032
- Table 20: United Kingdom Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 21: Germany Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 22: France Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 23: Italy Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 24: Spain Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 25: Russia Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 26: Benelux Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 27: Nordics Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 28: Rest of Europe Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 29: Global Inhalation CDMO Revenue million Forecast, by Type 2019 & 2032
- Table 30: Global Inhalation CDMO Revenue million Forecast, by Application 2019 & 2032
- Table 31: Global Inhalation CDMO Revenue million Forecast, by Country 2019 & 2032
- Table 32: Turkey Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 33: Israel Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 34: GCC Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 35: North Africa Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 36: South Africa Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 37: Rest of Middle East & Africa Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 38: Global Inhalation CDMO Revenue million Forecast, by Type 2019 & 2032
- Table 39: Global Inhalation CDMO Revenue million Forecast, by Application 2019 & 2032
- Table 40: Global Inhalation CDMO Revenue million Forecast, by Country 2019 & 2032
- Table 41: China Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 42: India Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 43: Japan Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 44: South Korea Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 45: ASEAN Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 46: Oceania Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
- Table 47: Rest of Asia Pacific Inhalation CDMO Revenue (million) Forecast, by Application 2019 & 2032
STEP 1 - Identification of Relevant Samples Size from Population Database



STEP 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note* : In applicable scenarios
STEP 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

STEP 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
Frequently Asked Questions
Related Reports
About Market Research Forecast
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.

