 Medical Wire for Medical Devices
Medical Wire for Medical DevicesMedical Wire for Medical Devices Report Probes the XXX million Size, Share, Growth Report and Future Analysis by 2033
Medical Wire for Medical Devices by Type (Overview: Global Medical Wire for Medical Devices Consumption Value, Stainless Steel Wire, Nitinol Wire, Platinum Wire, Tungsten Wire, Other), by Application (Overview: Global Medical Wire for Medical Devices Consumption Value, Medical Ultrasound Imaging Systems, Intracardiac Ablation Catheters, Endoscopic Therapeutic Devices, Other), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
Key Insights
The global medical wire for medical devices market size was valued at USD 1.43 billion in 2025 and is projected to grow from USD 1.53 billion in 2026 to USD 2.42 billion by 2033, exhibiting a CAGR of 6.1% during the forecast period (2026-2033). Medical grade wires are used for a variety of medical applications, including diagnostic imaging, ablation catheters, and endoscopic devices.
The market growth is attributed to factors such as the rising prevalence of chronic diseases, increasing adoption of minimally invasive surgical procedures, and technological advancements in medical devices. Furthermore, government initiatives and funding for healthcare infrastructure development in emerging economies are expected to create new opportunities for market growth. The market is segmented based on type and application. Stainless steel wire and nitinol wire are the dominant types of medical wire used in the market. Stainless steel wire is preferred due to its strength, corrosion resistance, and biocompatibility. Nitinol wire is used in applications requiring shape memory and superelasticity. Medical ultrasound imaging systems and intracardiac ablation catheters are the major applications of medical wire. Medical ultrasound imaging systems use medical wire for image guidance and probe positioning. Intracardiac ablation catheters use medical wire to deliver energy to the heart tissue for ablation procedures.

Medical Wire for Medical Devices Trends
The medical wire market is on a remarkable growth trajectory, propelled by soaring demand for minimally invasive medical procedures. This trend is evident in the widespread adoption of advanced medical techniques such as intracardiac ablation catheters, endoscopic therapeutic devices, and medical ultrasound imaging systems.
The increasing prevalence of cardiovascular diseases, neurovascular conditions, and digestive disorders has intensified the need for minimally invasive surgeries. These procedures offer numerous advantages over traditional open surgeries, including reduced pain, faster recovery times, and improved patient outcomes. Medical wires play a crucial role in these surgeries, providing structural support and guiding catheters and other medical devices to specific anatomical locations.
Driving Forces: What's Propelling the Medical Wire for Medical Devices
Rising Prevalence of Chronic Diseases: The increasing prevalence of chronic diseases such as diabetes, hypertension, and obesity has led to a surge in demand for medical devices and procedures to manage these conditions. Medical wires are widely used in devices such as pacemakers, stents, and catheters, which are essential for treating these conditions.
Technological Advancements: Technological advancements in medical imaging, surgical techniques, and device design have significantly enhanced the efficacy and safety of medical procedures. These advancements have created new applications for medical wires, particularly in minimally invasive surgeries and diagnostic imaging.

Challenges and Restraints in Medical Wire for Medical Devices
Strict Regulatory Standards: The medical device industry is heavily regulated to ensure patient safety and product quality. Medical wires must meet stringent regulatory requirements established by government agencies such as the FDA and ISO. Compliance with these regulations can increase manufacturing costs and delay the market entry of new products.
Competition from Low-Cost Manufacturers: The global medical wire market is highly competitive, with manufacturers from low-cost countries offering products at affordable prices. This competition can lead to price erosion and margin compression for established players.
Key Region or Country & Segment to Dominate the Market
Dominating Segment: Medical Ultrasound Imaging Systems
Medical ultrasound imaging systems are projected to dominate the global medical wire market, owing to their widespread use in diagnostic procedures such as prenatal imaging, cardiac imaging, and abdominal imaging. The increasing adoption of ultrasound-guided interventions and the expanding application areas of ultrasound technology are driving the growth of this segment.
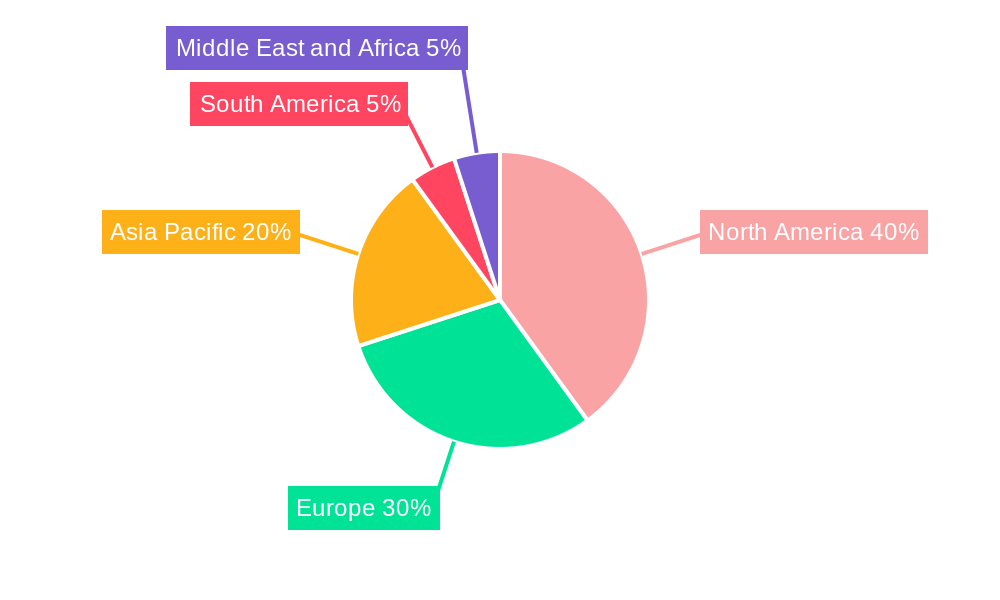
Dominating Region: North America
North America is expected to remain the dominant region in the medical wire market, driven by the presence of a large patient population, high healthcare spending, and the rapid adoption of advanced medical devices and technologies. The United States is the largest market within North America, with a significant demand for medical wires for cardiovascular and neurological applications.
Growth Catalysts in Medical Wire for Medical Devices Industry
Expanding Healthcare Access in Developing Countries: The increasing availability of healthcare services in developing countries is driving the demand for medical devices, including medical wires. Governments and healthcare organizations are investing in healthcare infrastructure and increasing access to medical care, which is expected to stimulate the growth of the medical wire market in these regions.
Emergence of New Technologies: Continuous advancements in medical technologies, such as the development of bioresorbable materials and biodegradable coatings, are creating new opportunities for medical wire applications. These technologies enhance the functionality and safety of medical devices, leading to improved patient outcomes.
Leading Players in the Medical Wire for Medical Devices
- Heraeus Group rel="nofollow"
- Alpha Wire rel="nofollow"
- SAB Bröckskes rel="nofollow"
- HEW rel="nofollow"
- Maeden rel="nofollow"
- LS Cable & System rel="nofollow"
- ZHAOLONG rel="nofollow"
- Proterial rel="nofollow"
- Plansee rel="nofollow"
- Nippon Steel SG Wire rel="nofollow"
- Ulbrich rel="nofollow"
- Haynes International rel="nofollow"
- Fort Wayne Metals Research Products rel="nofollow"
- ETCO rel="nofollow"
- Alleima rel="nofollow"
Significant Developments in Medical Wire for Medical Devices Sector
Medical wire manufacturers are actively engaged in research and development to improve the performance and functionality of their products. Recent developments include:
- Introduction of ultra-fine medical wires with improved flexibility and strength for use in minimally invasive surgeries.
- Development of biocompatible coatings to reduce the risk of infections and allergic reactions.
- Exploration of biodegradable materials to create medical wires that can dissolve over time, eliminating the need for removal surgeries.
Comprehensive Coverage Medical Wire for Medical Devices Report
This comprehensive report on the medical wire for medical devices market provides a comprehensive overview of the market trends, driving forces, challenges, and growth catalysts. It also offers detailed insights into the key regions, segments, and leading players in the market. The report is an invaluable resource for decision-makers in the medical device industry, including manufacturers, distributors, investors, and healthcare providers, who are looking to capitalize on the opportunities and navigate the challenges in this dynamic market.
Medical Wire for Medical Devices Segmentation
-
1. Type
- 1.1. Overview: Global Medical Wire for Medical Devices Consumption Value
- 1.2. Stainless Steel Wire
- 1.3. Nitinol Wire
- 1.4. Platinum Wire
- 1.5. Tungsten Wire
- 1.6. Other
-
2. Application
- 2.1. Overview: Global Medical Wire for Medical Devices Consumption Value
- 2.2. Medical Ultrasound Imaging Systems
- 2.3. Intracardiac Ablation Catheters
- 2.4. Endoscopic Therapeutic Devices
- 2.5. Other
Medical Wire for Medical Devices Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific
Medical Wire for Medical Devices REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|
Frequently Asked Questions
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Medical Wire for Medical Devices Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Type
- 5.1.1. Overview: Global Medical Wire for Medical Devices Consumption Value
- 5.1.2. Stainless Steel Wire
- 5.1.3. Nitinol Wire
- 5.1.4. Platinum Wire
- 5.1.5. Tungsten Wire
- 5.1.6. Other
- 5.2. Market Analysis, Insights and Forecast - by Application
- 5.2.1. Overview: Global Medical Wire for Medical Devices Consumption Value
- 5.2.2. Medical Ultrasound Imaging Systems
- 5.2.3. Intracardiac Ablation Catheters
- 5.2.4. Endoscopic Therapeutic Devices
- 5.2.5. Other
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Type
- 6. North America Medical Wire for Medical Devices Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Type
- 6.1.1. Overview: Global Medical Wire for Medical Devices Consumption Value
- 6.1.2. Stainless Steel Wire
- 6.1.3. Nitinol Wire
- 6.1.4. Platinum Wire
- 6.1.5. Tungsten Wire
- 6.1.6. Other
- 6.2. Market Analysis, Insights and Forecast - by Application
- 6.2.1. Overview: Global Medical Wire for Medical Devices Consumption Value
- 6.2.2. Medical Ultrasound Imaging Systems
- 6.2.3. Intracardiac Ablation Catheters
- 6.2.4. Endoscopic Therapeutic Devices
- 6.2.5. Other
- 6.1. Market Analysis, Insights and Forecast - by Type
- 7. South America Medical Wire for Medical Devices Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Type
- 7.1.1. Overview: Global Medical Wire for Medical Devices Consumption Value
- 7.1.2. Stainless Steel Wire
- 7.1.3. Nitinol Wire
- 7.1.4. Platinum Wire
- 7.1.5. Tungsten Wire
- 7.1.6. Other
- 7.2. Market Analysis, Insights and Forecast - by Application
- 7.2.1. Overview: Global Medical Wire for Medical Devices Consumption Value
- 7.2.2. Medical Ultrasound Imaging Systems
- 7.2.3. Intracardiac Ablation Catheters
- 7.2.4. Endoscopic Therapeutic Devices
- 7.2.5. Other
- 7.1. Market Analysis, Insights and Forecast - by Type
- 8. Europe Medical Wire for Medical Devices Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Type
- 8.1.1. Overview: Global Medical Wire for Medical Devices Consumption Value
- 8.1.2. Stainless Steel Wire
- 8.1.3. Nitinol Wire
- 8.1.4. Platinum Wire
- 8.1.5. Tungsten Wire
- 8.1.6. Other
- 8.2. Market Analysis, Insights and Forecast - by Application
- 8.2.1. Overview: Global Medical Wire for Medical Devices Consumption Value
- 8.2.2. Medical Ultrasound Imaging Systems
- 8.2.3. Intracardiac Ablation Catheters
- 8.2.4. Endoscopic Therapeutic Devices
- 8.2.5. Other
- 8.1. Market Analysis, Insights and Forecast - by Type
- 9. Middle East & Africa Medical Wire for Medical Devices Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Type
- 9.1.1. Overview: Global Medical Wire for Medical Devices Consumption Value
- 9.1.2. Stainless Steel Wire
- 9.1.3. Nitinol Wire
- 9.1.4. Platinum Wire
- 9.1.5. Tungsten Wire
- 9.1.6. Other
- 9.2. Market Analysis, Insights and Forecast - by Application
- 9.2.1. Overview: Global Medical Wire for Medical Devices Consumption Value
- 9.2.2. Medical Ultrasound Imaging Systems
- 9.2.3. Intracardiac Ablation Catheters
- 9.2.4. Endoscopic Therapeutic Devices
- 9.2.5. Other
- 9.1. Market Analysis, Insights and Forecast - by Type
- 10. Asia Pacific Medical Wire for Medical Devices Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Type
- 10.1.1. Overview: Global Medical Wire for Medical Devices Consumption Value
- 10.1.2. Stainless Steel Wire
- 10.1.3. Nitinol Wire
- 10.1.4. Platinum Wire
- 10.1.5. Tungsten Wire
- 10.1.6. Other
- 10.2. Market Analysis, Insights and Forecast - by Application
- 10.2.1. Overview: Global Medical Wire for Medical Devices Consumption Value
- 10.2.2. Medical Ultrasound Imaging Systems
- 10.2.3. Intracardiac Ablation Catheters
- 10.2.4. Endoscopic Therapeutic Devices
- 10.2.5. Other
- 10.1. Market Analysis, Insights and Forecast - by Type
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 Heraeus Group
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Alpha Wire
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 SAB Bröckskes
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 HEW
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Maeden
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 LS Cable & System
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 ZHAOLONG
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Proterial
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Plansee
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Nippon Steel SG Wire
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Ulbrich
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Haynes International
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.13 Fort Wayne Metals Research Products
- 11.2.13.1. Overview
- 11.2.13.2. Products
- 11.2.13.3. SWOT Analysis
- 11.2.13.4. Recent Developments
- 11.2.13.5. Financials (Based on Availability)
- 11.2.14 ETCO
- 11.2.14.1. Overview
- 11.2.14.2. Products
- 11.2.14.3. SWOT Analysis
- 11.2.14.4. Recent Developments
- 11.2.14.5. Financials (Based on Availability)
- 11.2.15 Alleima
- 11.2.15.1. Overview
- 11.2.15.2. Products
- 11.2.15.3. SWOT Analysis
- 11.2.15.4. Recent Developments
- 11.2.15.5. Financials (Based on Availability)
- 11.2.1 Heraeus Group
- Figure 1: Global Medical Wire for Medical Devices Revenue Breakdown (million, %) by Region 2024 & 2032
- Figure 2: Global Medical Wire for Medical Devices Volume Breakdown (K, %) by Region 2024 & 2032
- Figure 3: North America Medical Wire for Medical Devices Revenue (million), by Type 2024 & 2032
- Figure 4: North America Medical Wire for Medical Devices Volume (K), by Type 2024 & 2032
- Figure 5: North America Medical Wire for Medical Devices Revenue Share (%), by Type 2024 & 2032
- Figure 6: North America Medical Wire for Medical Devices Volume Share (%), by Type 2024 & 2032
- Figure 7: North America Medical Wire for Medical Devices Revenue (million), by Application 2024 & 2032
- Figure 8: North America Medical Wire for Medical Devices Volume (K), by Application 2024 & 2032
- Figure 9: North America Medical Wire for Medical Devices Revenue Share (%), by Application 2024 & 2032
- Figure 10: North America Medical Wire for Medical Devices Volume Share (%), by Application 2024 & 2032
- Figure 11: North America Medical Wire for Medical Devices Revenue (million), by Country 2024 & 2032
- Figure 12: North America Medical Wire for Medical Devices Volume (K), by Country 2024 & 2032
- Figure 13: North America Medical Wire for Medical Devices Revenue Share (%), by Country 2024 & 2032
- Figure 14: North America Medical Wire for Medical Devices Volume Share (%), by Country 2024 & 2032
- Figure 15: South America Medical Wire for Medical Devices Revenue (million), by Type 2024 & 2032
- Figure 16: South America Medical Wire for Medical Devices Volume (K), by Type 2024 & 2032
- Figure 17: South America Medical Wire for Medical Devices Revenue Share (%), by Type 2024 & 2032
- Figure 18: South America Medical Wire for Medical Devices Volume Share (%), by Type 2024 & 2032
- Figure 19: South America Medical Wire for Medical Devices Revenue (million), by Application 2024 & 2032
- Figure 20: South America Medical Wire for Medical Devices Volume (K), by Application 2024 & 2032
- Figure 21: South America Medical Wire for Medical Devices Revenue Share (%), by Application 2024 & 2032
- Figure 22: South America Medical Wire for Medical Devices Volume Share (%), by Application 2024 & 2032
- Figure 23: South America Medical Wire for Medical Devices Revenue (million), by Country 2024 & 2032
- Figure 24: South America Medical Wire for Medical Devices Volume (K), by Country 2024 & 2032
- Figure 25: South America Medical Wire for Medical Devices Revenue Share (%), by Country 2024 & 2032
- Figure 26: South America Medical Wire for Medical Devices Volume Share (%), by Country 2024 & 2032
- Figure 27: Europe Medical Wire for Medical Devices Revenue (million), by Type 2024 & 2032
- Figure 28: Europe Medical Wire for Medical Devices Volume (K), by Type 2024 & 2032
- Figure 29: Europe Medical Wire for Medical Devices Revenue Share (%), by Type 2024 & 2032
- Figure 30: Europe Medical Wire for Medical Devices Volume Share (%), by Type 2024 & 2032
- Figure 31: Europe Medical Wire for Medical Devices Revenue (million), by Application 2024 & 2032
- Figure 32: Europe Medical Wire for Medical Devices Volume (K), by Application 2024 & 2032
- Figure 33: Europe Medical Wire for Medical Devices Revenue Share (%), by Application 2024 & 2032
- Figure 34: Europe Medical Wire for Medical Devices Volume Share (%), by Application 2024 & 2032
- Figure 35: Europe Medical Wire for Medical Devices Revenue (million), by Country 2024 & 2032
- Figure 36: Europe Medical Wire for Medical Devices Volume (K), by Country 2024 & 2032
- Figure 37: Europe Medical Wire for Medical Devices Revenue Share (%), by Country 2024 & 2032
- Figure 38: Europe Medical Wire for Medical Devices Volume Share (%), by Country 2024 & 2032
- Figure 39: Middle East & Africa Medical Wire for Medical Devices Revenue (million), by Type 2024 & 2032
- Figure 40: Middle East & Africa Medical Wire for Medical Devices Volume (K), by Type 2024 & 2032
- Figure 41: Middle East & Africa Medical Wire for Medical Devices Revenue Share (%), by Type 2024 & 2032
- Figure 42: Middle East & Africa Medical Wire for Medical Devices Volume Share (%), by Type 2024 & 2032
- Figure 43: Middle East & Africa Medical Wire for Medical Devices Revenue (million), by Application 2024 & 2032
- Figure 44: Middle East & Africa Medical Wire for Medical Devices Volume (K), by Application 2024 & 2032
- Figure 45: Middle East & Africa Medical Wire for Medical Devices Revenue Share (%), by Application 2024 & 2032
- Figure 46: Middle East & Africa Medical Wire for Medical Devices Volume Share (%), by Application 2024 & 2032
- Figure 47: Middle East & Africa Medical Wire for Medical Devices Revenue (million), by Country 2024 & 2032
- Figure 48: Middle East & Africa Medical Wire for Medical Devices Volume (K), by Country 2024 & 2032
- Figure 49: Middle East & Africa Medical Wire for Medical Devices Revenue Share (%), by Country 2024 & 2032
- Figure 50: Middle East & Africa Medical Wire for Medical Devices Volume Share (%), by Country 2024 & 2032
- Figure 51: Asia Pacific Medical Wire for Medical Devices Revenue (million), by Type 2024 & 2032
- Figure 52: Asia Pacific Medical Wire for Medical Devices Volume (K), by Type 2024 & 2032
- Figure 53: Asia Pacific Medical Wire for Medical Devices Revenue Share (%), by Type 2024 & 2032
- Figure 54: Asia Pacific Medical Wire for Medical Devices Volume Share (%), by Type 2024 & 2032
- Figure 55: Asia Pacific Medical Wire for Medical Devices Revenue (million), by Application 2024 & 2032
- Figure 56: Asia Pacific Medical Wire for Medical Devices Volume (K), by Application 2024 & 2032
- Figure 57: Asia Pacific Medical Wire for Medical Devices Revenue Share (%), by Application 2024 & 2032
- Figure 58: Asia Pacific Medical Wire for Medical Devices Volume Share (%), by Application 2024 & 2032
- Figure 59: Asia Pacific Medical Wire for Medical Devices Revenue (million), by Country 2024 & 2032
- Figure 60: Asia Pacific Medical Wire for Medical Devices Volume (K), by Country 2024 & 2032
- Figure 61: Asia Pacific Medical Wire for Medical Devices Revenue Share (%), by Country 2024 & 2032
- Figure 62: Asia Pacific Medical Wire for Medical Devices Volume Share (%), by Country 2024 & 2032
- Table 1: Global Medical Wire for Medical Devices Revenue million Forecast, by Region 2019 & 2032
- Table 2: Global Medical Wire for Medical Devices Volume K Forecast, by Region 2019 & 2032
- Table 3: Global Medical Wire for Medical Devices Revenue million Forecast, by Type 2019 & 2032
- Table 4: Global Medical Wire for Medical Devices Volume K Forecast, by Type 2019 & 2032
- Table 5: Global Medical Wire for Medical Devices Revenue million Forecast, by Application 2019 & 2032
- Table 6: Global Medical Wire for Medical Devices Volume K Forecast, by Application 2019 & 2032
- Table 7: Global Medical Wire for Medical Devices Revenue million Forecast, by Region 2019 & 2032
- Table 8: Global Medical Wire for Medical Devices Volume K Forecast, by Region 2019 & 2032
- Table 9: Global Medical Wire for Medical Devices Revenue million Forecast, by Type 2019 & 2032
- Table 10: Global Medical Wire for Medical Devices Volume K Forecast, by Type 2019 & 2032
- Table 11: Global Medical Wire for Medical Devices Revenue million Forecast, by Application 2019 & 2032
- Table 12: Global Medical Wire for Medical Devices Volume K Forecast, by Application 2019 & 2032
- Table 13: Global Medical Wire for Medical Devices Revenue million Forecast, by Country 2019 & 2032
- Table 14: Global Medical Wire for Medical Devices Volume K Forecast, by Country 2019 & 2032
- Table 15: United States Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 16: United States Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 17: Canada Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 18: Canada Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 19: Mexico Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 20: Mexico Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 21: Global Medical Wire for Medical Devices Revenue million Forecast, by Type 2019 & 2032
- Table 22: Global Medical Wire for Medical Devices Volume K Forecast, by Type 2019 & 2032
- Table 23: Global Medical Wire for Medical Devices Revenue million Forecast, by Application 2019 & 2032
- Table 24: Global Medical Wire for Medical Devices Volume K Forecast, by Application 2019 & 2032
- Table 25: Global Medical Wire for Medical Devices Revenue million Forecast, by Country 2019 & 2032
- Table 26: Global Medical Wire for Medical Devices Volume K Forecast, by Country 2019 & 2032
- Table 27: Brazil Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 28: Brazil Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 29: Argentina Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 30: Argentina Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 31: Rest of South America Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 32: Rest of South America Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 33: Global Medical Wire for Medical Devices Revenue million Forecast, by Type 2019 & 2032
- Table 34: Global Medical Wire for Medical Devices Volume K Forecast, by Type 2019 & 2032
- Table 35: Global Medical Wire for Medical Devices Revenue million Forecast, by Application 2019 & 2032
- Table 36: Global Medical Wire for Medical Devices Volume K Forecast, by Application 2019 & 2032
- Table 37: Global Medical Wire for Medical Devices Revenue million Forecast, by Country 2019 & 2032
- Table 38: Global Medical Wire for Medical Devices Volume K Forecast, by Country 2019 & 2032
- Table 39: United Kingdom Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 40: United Kingdom Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 41: Germany Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 42: Germany Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 43: France Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 44: France Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 45: Italy Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 46: Italy Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 47: Spain Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 48: Spain Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 49: Russia Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 50: Russia Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 51: Benelux Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 52: Benelux Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 53: Nordics Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 54: Nordics Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 55: Rest of Europe Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 56: Rest of Europe Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 57: Global Medical Wire for Medical Devices Revenue million Forecast, by Type 2019 & 2032
- Table 58: Global Medical Wire for Medical Devices Volume K Forecast, by Type 2019 & 2032
- Table 59: Global Medical Wire for Medical Devices Revenue million Forecast, by Application 2019 & 2032
- Table 60: Global Medical Wire for Medical Devices Volume K Forecast, by Application 2019 & 2032
- Table 61: Global Medical Wire for Medical Devices Revenue million Forecast, by Country 2019 & 2032
- Table 62: Global Medical Wire for Medical Devices Volume K Forecast, by Country 2019 & 2032
- Table 63: Turkey Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 64: Turkey Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 65: Israel Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 66: Israel Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 67: GCC Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 68: GCC Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 69: North Africa Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 70: North Africa Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 71: South Africa Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 72: South Africa Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 73: Rest of Middle East & Africa Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 74: Rest of Middle East & Africa Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 75: Global Medical Wire for Medical Devices Revenue million Forecast, by Type 2019 & 2032
- Table 76: Global Medical Wire for Medical Devices Volume K Forecast, by Type 2019 & 2032
- Table 77: Global Medical Wire for Medical Devices Revenue million Forecast, by Application 2019 & 2032
- Table 78: Global Medical Wire for Medical Devices Volume K Forecast, by Application 2019 & 2032
- Table 79: Global Medical Wire for Medical Devices Revenue million Forecast, by Country 2019 & 2032
- Table 80: Global Medical Wire for Medical Devices Volume K Forecast, by Country 2019 & 2032
- Table 81: China Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 82: China Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 83: India Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 84: India Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 85: Japan Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 86: Japan Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 87: South Korea Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 88: South Korea Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 89: ASEAN Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 90: ASEAN Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 91: Oceania Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 92: Oceania Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
- Table 93: Rest of Asia Pacific Medical Wire for Medical Devices Revenue (million) Forecast, by Application 2019 & 2032
- Table 94: Rest of Asia Pacific Medical Wire for Medical Devices Volume (K) Forecast, by Application 2019 & 2032
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|
STEP 1 - Identification of Relevant Samples Size from Population Database



STEP 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note* : In applicable scenarios
STEP 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

STEP 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
Related Reports
About Market Research Forecast
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.

